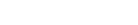Contract API & intermediate manufacturing
Commercial manufacturing capabilities for glyco-therapeutics
Commercial manufacturing is the key step in advancing your drug candidate from the lab to the market. GlyTech not only provides R&D support for the development of unique glycosylated drug candidates – we are also committed to supporting their manufacturing throughout the whole product life cycle, including large scale production of non-GMP and GMP active pharmaceutical ingredients (APIs) for pre-clinical tests, clinical trials and commercialization.
We are the experts in producing oligosaccharide/glycan-based reagents, intermediates, and APIs, and ready to be of service to your drug development.
Flexible manufacturing model
Intermediate and API manufacturing at GlyTech, Inc.
GlyTech is the world leader in the synthetic manufacturing of homogenously glycan-modified APIs such as glycopeptides and small glycoproteins. Our team has wide-ranging expertise in the major peptide technologies and nearly 20 years’ experience at the forefront of large-scale chemical glycosylation.
This enables us to manufacture high quality glycosylated drug substances under GMP conditions, ready for formulation to create high value final drug products for clinical trials and commercialization. We’re also ideally placed to provide non-GMP grade APIs for early-stage projects, starting at a few milligrams in scale.
Depending on the nature of the drug substance, the manufacturing process of an API can involve the use of many different and complex technologies and procedures to change each intermediate compound into the next until the final API is obtained.
GlyTech’s chemical synthesis-based technologies can be used to manufacture glycan reagents, glycosylated materials and glycoconjugate intermediates that can be combined with our partners’ technology platforms or integrated into their manufacturing processes. We’re committed to helping you produce your target APIs without the worries associated with glycoform variation.
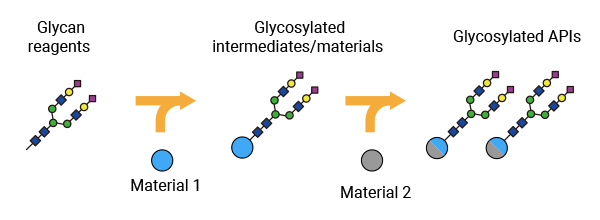
GlyTech’s manufacturing model
Our chemical glycosylation technology is compatible with conventional peptide synthesis methods, including solid-phase peptide synthesis (SPPS) and liquid-phase peptide synthesis (LPPS), and with a wide range of conjugation chemistries.
This makes it easy for us to synergize our technology with existing manufacturing methods and facilities and expand our large-scale manufacturing capabilities in cooperation with our global manufacturing partners.
We’re experienced in glycosylated API synthesis method development and scale-up, and in the transfer of these methods to manufacturing partners. We take responsibility for quality assurance (QA) during the manufacturing process and help our partners develop analytical methods and impurity and stability studies to ensure product quality.
Case study: GT-02037 (Phase I clinical trial implemented)
- In-house development and scale-up of manufacturing method
- Homogeneous glycopeptide API manufactured by CMO partner using traditional SPPS method and our glycan material
- QA, impurity & stability studies, and IND application carried out by GlyTech
- Phase 1 clinical trial successfully completed
We offer:
- GMP and non-GMP manufacturing of glycosylated APIs and glycosylated intermediates
- Up to kilogram scale glycan manufacturing
- Process development, including scale-up methods
- Analytical method development
- Impurity and stability studies
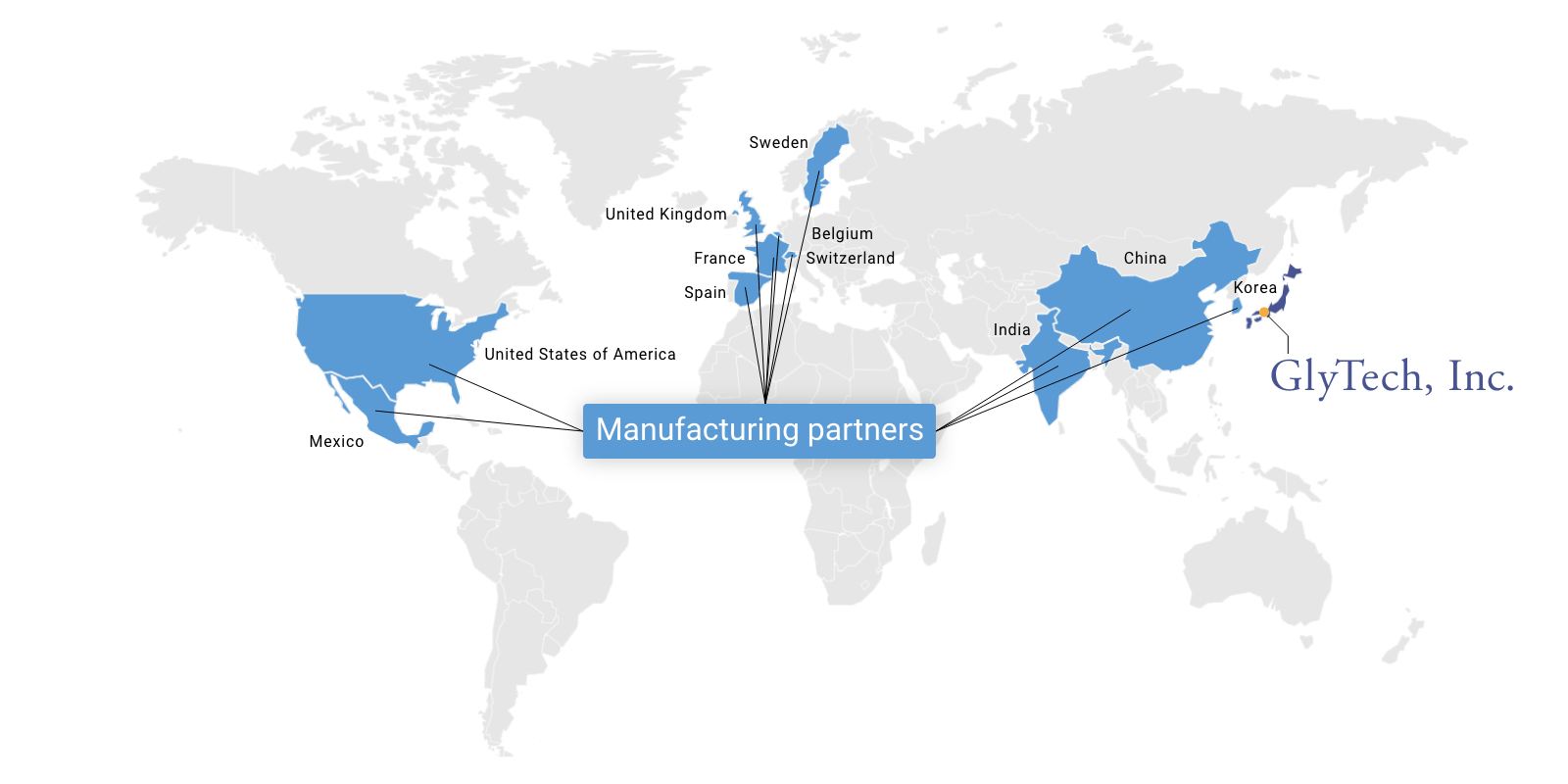
Global manufacturing network
The quality and purity of active pharmaceutical ingredients (APIs) must be strictly managed according to cGMP regulations during their manufacturing. API manufacturing is a sophisticated process that requires vast knowledge and experience.
At GlyTech, we have worked closely with several leading API manufacturers (CMOs) and built up a global manufacturing network. Our partners are proven experts in API manufacturing and highly experienced in pharmaceutical production and regulation compliance.
Leveraging the advantages of these manufacturing partners allows us to reliably provide high quality glycosylated APIs and glycosylated materials from the most convenient base, reducing the time and cost to our clients and the risk of unexpected delays.